Fractional distillation
|
|
Fractional distillation is the separation of a mixture of compounds by their boiling point, by heating to high enough temperatures.
 |
| Contents |
Fractional Distillation in a Laboratory
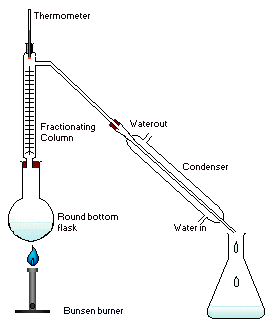
Apparatus
- anti-bumping granules
- rubber bungs (unless quickfit apparatus is used)
Method
As an example, consider the distillation of a mixture of water and ethanol. Ethanol boils at 78.5°C whilst water boils at 100°C. So by gently heating the mixture, the most volatile component will boil off first. Some mixtures form azeotropes, where the mixture boils at a lower temperature than either component. In this example, a mixture of 95% ethanol and 5% water boils at 78.2°C, being more volatile than pure ethanol, so the ethanol cannot be completely purified by distillation.
The apparatus is assembled as in the diagram. The mixture is put into the round bottomed flask along with a few anti bumping granules, and the fractionating column is fitted into the top. As the mixture boils, vapor rises up the column. The vapor condenses on the glass platforms inside the column, and runs back down into the liquid below, refluxing distillate. Under proper operating conditions, only the most volatile of the vapors stays in gaseous form all the way to the top. The vapor at the top of the column, then passes into the condenser, which cools it down until it liquefies. The condensate that was initially very close to the azeotrope composition becomes gradually richer in water. The process continues until all the ethanol boils out of the mixture. This point can be recognized by the sharp rise in temperature shown on the thermometer.
Industrial uses of Fractional Distillation
Main article: Oil refinery
The most important industrial application of fractional distillation is the distillation of crude oil. The process is similar in principle to the laboratory method described above except for scale, continuous feed and operation, and the fact that crude oil has many different compounds mixed together. The fractionating column has outlets at regular intervals up the column which allow the different fractions to run out at different temperatures, with the highly volatile gases coming out the topmost outlet graduating to the less volatile road tar (bitumen) coming out at the bottom.
Fractional distillation process is also used in air separation, producing liquid oxygen, liquid nitrogen, and high purity argon. Distillation of chlorosilanes also enable the production of high-purity silicon for use as a semiconductor.
