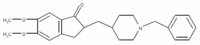
Donepezil
|
|
| 2,3-dihydro-5,6-dimethoxy-2- ((1-(phenylmethyl)-4-piperidinyl) methyl)-1H-inden-1-one, | |
| CAS number 120014-06-4 | ATC code N06DA02 |
| Chemical formula | C24H29NO3 |
| Molecular weight | 379.497 |
| Bioavailability | ? |
| Metabolism | ? |
| Elimination half-life | ? |
| Excretion | ? |
| Pregnancy category | ? |
| Legal status | ? |
| Routes of administration | Oral tablet, 5 & 10 mg |
Donepezil, marketed under the trade name Aricept® (Pfizer), is a centrally acting reversible acetyl cholinesterase inhibitor. Its main therapeutic use is in the treatment of Alzheimer's disease where it is used to increase cortical acetylcholine. It is well absorbed in the gut with an oral bioavailability of 100% and easily crosses the blood-brain barrier. Because it has a half life of about 70 hours, it can be taken once a day.
As of the 22 March 2005, the UK National Institute for Clinical Excellence (NICE) withdrew its recommendation for use of the drug for mild-to-moderate AD, on the basis that there is no significant improvement in functional outcome; of quality of life or of behavioral symptoms.
Sources
- Brenner, G. M. (2000). Pharmacology. Philadelphia, PA: W.B. Saunders Company. ISBN 0-7216-7757-6
- Canadian Pharmacists Association (2000). Compendium of Pharmaceuticals and Specialties (25th ed.). Toronto, ON: Webcom. ISBN 0-919115-76-4