Cellular respiration
|
|
Cellular respiration is, in its broadest definition, the process in which the chemical bonds of energy-rich molecules such as glucose are converted into energy usable for life processes. Oxidation of organic material—in a bonfire, for example—is an exothermic reaction that releases a large amount of energy rather quickly. The overall equation for the oxidation of glucose is:
- C6H12O6 + 6O2 → 6CO2 + 6H2O + energy
In cellular respiration, this oxidation process is broken down into two basic metabolic pathways: glycolysis, anaerobic respiration or aerobic respiration.
| Contents |
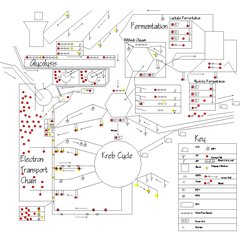
Glycolysis
Main article: Glycolysis
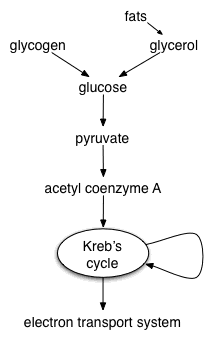
Glycolysis is a metabolic pathway that is found in all living organisms and does not require oxygen. The process converts one molecule of glucose into two molecules of pyruvate, and makes energy in the form of two molecules of ATP. Glycolysis takes place in the cytoplasm of the cell. The overall reaction can be expressed this way:
- Glucose + 2 NAD+ + 2 ATP + 2 Pi → 2 NADH + 2 pyruvate + 4 ATP + 2 H2O + 4 H+
The individual steps of the conversion of glucose into pyruvate are (in brief):
- A glucose molecule from the hydrolysation of starch or glycogen is phosphorylated using one ATP molecule to give glucose-6-phosphate.
- The glucose-6-phosphate is converted to fructose-6-phosphate by isomerisation.
- Fructose-6-phosphate is again phosphorylated to give fructose-1,6-diphosphate with the use of another ATP molecule.
- Next, the fructose-1,6-diphosphate is then lysed into two molecules of 3-carbon sugar (dihydroxyacetone phosphate and glyceraldehyde-3-phosphate) which are interconvertible.
- The 3-carbon sugars are dehydrogenated and inorganic phosphate is added to them, forming two molecules of 1,3 diphosphoglycerate.
- The hydrogen is used to oxidise two molecules of NAD, a hydrogen carrier, to give NADH+H+. NADH+H+ later proceeds to the mitochondria for use in the electron transport chain.
- The two molecules of 1,3 diphosphoglycerate lose two phosphate groups to form two molecules of glycerate-3-phosphate (3-phosphoglycerate), converting two molecules of ADP to ATP.
- The two molecules of glycerate-3-phosphate again lose phosphate forming two molecules of pyruvate, with the production of another two ATP molecules (for a net gain of 2 ATP).
Breakdown of pyruvate
There are now two ways to break down the resulting pyruvate:
Aerobic respiration (Cellular Respiration)
Aerobic respiration requires oxygen in order to generate energy. It is the preferred method of pyruvate breakdown. A molecule of pyruvate acid travels into a mitochondrion entering the Krebs cycle. In this process it is broken down producing energy in the form of ATP (which travels to the cell), NADH and FADH2 which travel to the electron transport chain. In this process, an electron is transferred from an energy-rich atom (such as a carbon atom in an organic molecule) to an oxygen atom, via an electron transport chain. Oxygen serves as the "terminal electron acceptor" in the electron transport chain. In the process, it yields 36 ATP molecules via the diffusion of hydrogen atoms through an ATP synthase, as well as carbon dioxide and water. This makes for a total gain of 38 ATP molecules during cellular respiration under optimal conditions; however, such conditions are generally not realized due to such losses as the cost of moving pyruvate into mitochondria. This takes place in the mitochondria in eukaryotic cells, and at the cell membrane in prokaryotic cells.
Aerobic metabolism is rather more efficient than anaerobic metabolism. It actually starts off with the Glycolysis process of anaerobic metabolism, and then continues with the krebs cycle and oxydative phosphorylation.
Glycolysis
Usually whatever is being metabolised is first converted to Acetyl-CoA, for sugars this would be by sugar->pyruvate by glycolysis then pyruvate->Acetyl-CoA. This process yields a net gain in energy in the form of ATP. If no oxygen is present, then the proces goes no further. (see also: anaerobic metabolism ) The word equation for aerobic respiration is glucose+oxygen=carbon dioxide+water+energy
Krebs cycle
When oxygen is present, Acetyl-CoA enters the citric acid cycle, and gets converted to CO2 while at the same time converting NAD + H to NADH. NADH can be used by several different processes to create further ATP. Commonly Oxidative phosphorylation in the mitochondria, where oxygen is used to split the H off of NADH, and to produce further ATP in the process.
Anaerobic respiration (Fermentation)
"Anaerobic respiration" It does not require oxygen. True anaerobic respiration involves an electron acceptor other than oxygen. Bacteria are capable of using a wide variety of compounds as terminal electron acceptors in respiration: nitrogenous compounds (such as nitrates and nitrites), sulfur compounds (such as sulfates, sulfites, sulfur dioxide, and elemental sulfur), carbon dioxide, iron compounds, manganese compounds, cobalt compounds, and uranium compounds.
However, none of these alternative electron acceptors yields as much energy from respiration as does oxygen. In environments where oxygen is present, typically only aerobic respiration will occur.
Fermentation is a process in which pyruvate is partially broken down, but there is no Krebs cycle and no production of ATP by an electron transport chain. Fermentations of various kinds produce a number of different compounds. Textbook examples of fermentation products are ethanol (drinkable alcohol), lactic acid, and hydrogen. However, more exotic compounds can be produced by fermentation, such as butyric acid and acetone.
Although fermentation produces no ATP, it is useful to the cell because it regenerates nicotinamide adenine dinucleotide (NAD+), which is consumed by glycolysis.
- Ethanol fermentation (done by yeast and some types of bacteria) breaks the pyruvate down into ethanol, carbon dioxide, and water. It is important in bread making, brewing, and wine making.
- Lactic acid fermentation breaks the pyruvate down into lactic acid, carbon dioxide, and water. It occurs in the muscles of animals when they need energy faster than the blood can supply oxygen. It also occurs in some bacteria. It is this type of bacteria that convert lactose into lactic acid in yogurt giving it its sour taste.
Fermentation products contain chemical energy that cannot be further broken down by fermentation, making fermentation less efficient than respiration. Fermentation releases a total of two ATP molecules per molecule of glucose (compare to the approximately 38 of aerobic respiration).
See also
External links
- A detailed diagram of glycolysis (http://www.people.virginia.edu/~rjh9u/glycol.html)
- Chart of Important Metabolic Products (http://departments.oxy.edu/biology/bio130/lectures_2000/metabolic_products.htm)da:Aerob respiration
de:Zellatmung fr:Respiration aérobie es:Respiración celular fr:Respiration cellulaire ja:呼吸 he:נשימה תאית pl:Oddychanie komórkowe fi:Soluhengitys