Bisphosphonate
|
|
In pharmacology, bisphosphonates (also called: diphosphonates) is a class of drugs that inhibits the resorption of bone. Its uses are the prevention and treatment of osteoporosis, osteitis deformans ("Paget's disease of bone"), bone metastasis (with or without hypercalcemia), multiple myeloma and other conditions that feature bone fragility.
| Contents |
Basic structure
All bisphosphonate drugs share a common "backbone":
Missing image
Bisphosphonate_basic_structure.png
basic structure of bisphosphonates
The two PO3 (phosphate) groups covalently linked to carbon determine both the name "bisphosphonate" and the function of the drugs.
The long side chain (R2 in the diagram) determines the chemical properties, the mode of action and the strength of bisphosphonate drugs. The short side chain (R1) mainly influences chemical properties and pharmacokinetics.
Pharmacokinetics
Of the bisphosphonate that is resorbed (from oral preparation) or infused (for intravenous drugs), about 50% is excreted unchanged by the kidney. The remainder has a very high affinity for bone tissue, and is rapidly absorbed onto the bone surface.
Side effects
- Oral bisphosphonates can give stomach upset and erosions of the esophagus, which is the main problem of oral preparation. This can be prevented by remaining seated upright for 30 minutes after taking the medication.
- There is a slightly increased risk for electrolyte disturbances, but not enough to warrant regular monitoring.
- In chronic renal failure, the drugs are excreted much slower, and dose adjustment is required.
- Some believe that bisphosphonates can cause osteonecrosis, a serious bone disorder. Genetic variation in a person's blood-clotting mechanisms can greatly increase this risk.
Classes of bisphosphonates
There are two classes of bisphosphonate: the N-containing and non-N-containing bisphosphonates.
Bisphosphonate_side_chains.png
side chains of bisphosphonate molecules
Non-nitrogenous
Non-N-containing bisphosphonates:
- Etidronate (Didronel®) - 1 (potency relative to that of etidronate)
- Clodronate (Bonefos®, Loron®) - 10
- Tiludronate (Skelid®) - 10
Nitrogenous
N-containing bisphosphonates:
- Pamidronate (APD, Aredia®) - 100
- Neridronate - 100
- Olpadronate - 500
- Alendronate (Fosamax®) - 500
- Ibandronate (Bondronat®) - 1000
- Risedronate (Actonel®) - 2000
- Zoldronate (Zometa®) - 10000
Mechanism of action
Bisphosphonates, when attached to bone tissue, are "ingested" by osteoclasts, the bone cell that breaks down bone tissue. The two types of bisphonates work differently in killing osteoclast cells.
Non-nitrogenous
The non-nitrogenous bisphosphonates are metabolised in the cell to compounds that compete with adenosine triphosphate (ATP) in the cellular energy metabolism. The osteoclast initiates apoptosis and dies, leading to an overall decrease in the breakdown of bone.
Nitrogenous
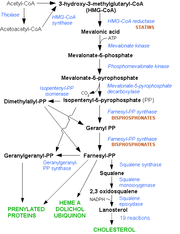
Nitrogenous bisphosphonates act on bone metabolism by bearing a chemical similarity to pyridoxal phosphate (PPi), which is a phosphate donor in the HMG-CoA reductase pathway.
The HMC CoA-reductase pathway is the metabolic pathway for a number of essential cellular compounds. The most prominent one is cholesterol. For osteoclasts, however, two other metabolites (farnesol and geranylgeraniol) are essential to connect some small proteins (ras protein, rho protein) to the cell membrane, as these confer hydrophobic properties when attached to these otherwise hydrophilic molecules (see "lipid anchored protein" for the principles of this phenomenon).
The rho protein connects the cytoskeleton to the cell membrane. In the case of the osteoclast, the cytoskeleton is essential to maintain the "ruffled border", the area with which the cell makes contact with bone and breaks down bone tissue. With the ruffled border compromised, the osteoclast initiates apoptosis, netting a decrease in bone turnover.
Statins are another class of drugs that inhibit the HMG-CoA reductase pathway. They are not specific for bone, and although some studies have reported a decreased rate of fracture (an indicator of osteoporosis) in statin users, there is no point in prescribing statins for osteoporosis patients.