Isopropyl alcohol
|
|
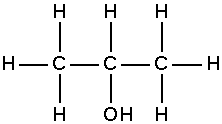
Isopropyl alcohol or isopropanol is a common name for 2-propanol, an alcohol which is the main component of rubbing alcohol. Its chemical structure is:
Its formula is C3H8O or more descriptively, CH3.CHOH.CH3.
The alcohol-based sterilizing swabs used to clean skin before injections typically contain a 72% solution of isopropanol in water.
Isopropyl alcohol is also commonly used as a cleaner and solvent in industry. It is also used as a gasoline additive for dissolving water or ice in fuel lines.
- Molecular Weight: 60.10
- Freezing Point: −89�C
- Boiling Point: 83�C
Density: 804.13 kg/m3 at 25�C, or approximately 80% the density of water.
from http://www.epa.gov/grtlakes/seahome/housewaste/house/isoalco.htm:
Isopropyl alcohol, also known as isopropanol, is a colorless liquid with an odor reminiscent of ethanol or acetone. It is highly flammable. Isopropyl alcohol is found in alcohol sponges, cleaning agents, and rubbing alcohol (though some rubbing alcohols contain ethanol), and is a good disinfectant. Most rubbing alcohol contains 70% isopropyl alcohol. Poisoning can occur through skin absorption, oral ingestion, or inhalation. Symptoms from ingestion, inhalation or absorption of large quantities include flushing, headache, dizziness, mental depression, nausea, vomiting, anesthesia, and coma. Alcohol baths or sponges to soothe a fever can lead to acute poisoning through skin absorption or inhalation. Instead, the Regional Poison Center suggests using tepid water as a sponge bath to fight fever.
Use: Wear protective gloves when using (see "Household Safety Equipment"). Use in well-ventilated areas.
Storage: Keep out of the reach of children and pets. Make sure lid is tightly capped. Store away from sources of flame or ignition.
Disposal: Flush down drain with plenty of water. If you have a sewage tank or lagoon, dispose of small quantities over a number of days.
External links
- Isopropyl Alcohol information page (http://www.embbs.com/cr/alc/alc5.html)
- Mass, Weight, Density or Specific Gravity of Liquids (http://www.simetric.co.uk/si_liquids.htm)