Olivine
|
|
The mineral olivine is a magnesium iron silicate with the formula (Mg,Fe)2SiO4 in which the ratio of magnesium and iron varies between the two endmembers of the series: forsterite (Mg-rich) and fayalite (Fe-rich). It gives its name to the group of minerals with a related structure (the olivine group) which includes monticellite and kirschsteinite. Olivine occurs in both mafic and ultramafic igneous rocks, and as a primary mineral in certain metamorphic rocks. It is one of the most common minerals on Earth.
| Contents |
Identification and paragenesis
Olivine is usually colored olive-green (hence the name), though it may be reddish from the oxidation of iron. It has a conchoidal fracture and is rather brittle. The hardness of olivine is 6.5-7, its relative density is 3.27-3.37 and it has a vitreous lustre. It is transparent to translucent.
Transparent olivine is sometimes used as a gemstone, often called peridot, the French word for olivine. It is also called chrysolite from the Greek words for gold and stone.
Olivine crystallizes from magma that is rich in magnesium and low in silica, which forms mafic to ultramafic rocks such as gabbro, basalt, peridotite, and dunite. The metamorphism of impure dolomite or other sedimentary rocks with high magnesium and low silica content also seems to produce Mg-rich olivine, or forsterite. Olivine or high pressure structural variants also constitute over 50% of the Earth's upper mantle making it one of the Earth's most common minerals by volume. Olivine has also been discovered in meteorites, on Mars, and on Earth's moon.
Crystal structure
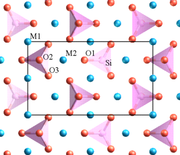
Minerals in the olivine group crystallize in the orthorhombic system (space group Pbnm) with isolated silicate tetrahedra meaning that olivine is a nesosilicate. In an alternative view, the atomic structure can be described as a hexagonal close packed array of oxygen ions with half of the octahedral sites occupied with magnesium or iron ions and one eighths of the tetrahedral sites occupied by silicon ions.
There are three distinct oxygen sites (marked O1, O2 and O3 in figure 1), two distinct metal sites (m1 and M2) and only one distinct silicon site. O1, O2, M2 and Si all lie on mirror planes while M1 exists on an inversion center. O3 lies in a general position.
High pressure polymorphs and phase diagram
At the high temperatures and pressures found at depth within the Earth the olivine structure is no longer stable. Below depths of about 400 km olivine undergoes a phase transition to the sorosilicate wadslyite and at about 520 km depth wadslyite transforms into ringwoodite which has the spinel structure. These phase transitions lead to a discontinuous increase in the density of the Earth's mantle that can be observed by seismic methods.
The pressure at which these phase transitions occour depends on temperature and iron content (Deer et al. 1992). At 800 °C the pure magnesium end member, forsterite, transforms to wadslyite at 11.8 gigapascals (118 kbar) and to ringwoodite at pressures above 14 GPa (140 kbar). Increasing the iron content decreases the pressure of the phase transition and narrows the wadslyite stability field. At about 0.8 mole fraction fayalite, olivine transforms directly to ringwoodite over the pressure range 10–11.5 GPa (100–115 kbar). Fayalite transforms to Fe2SiO4 spinel at pressures below 5 GPa (50 kbar). Increasing the temperature increases the pressure of these phase transitions.
See also
References
- Hurlbut, Cornelius S., 1966 pr, Dana's Manual of Mineralogy, 17th ed., ISBN 0471032883
- Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., ISBN 0471805807
- Deer, W. A., Howie, R. A., and Zussman, J. (1992). An introduction to the rock-forming minerals (2nd ed.). Harlow: Longman ISBN 0-582-30094-0de:Olivin
eo:Olivino he:אוליבין nl:Olivijn ja:カンラン石 pl:Oliwin pt:Olivina sk:Olivín