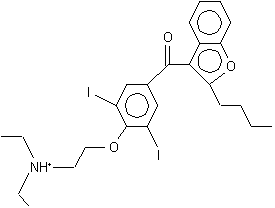
Amiodarone
|
|
|
2-butylbenzofuran-3-yl 4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone hydrochloride | |
| Empiric formula | C25H29I2NO3 . HCl |
| Molecular weight | 681.78 |
| Bioavailability (Oral) | 22 - 95% |
| Metabolism | Liver |
| Elimination half life (Oral) | 13 to 103 days |
| Elimination half life (IV) | 3.2-80 hours |
| Excretion | Epithelial cells |
| Pregnancy category | D (US) |
Amiodarone is a class III antiarrhythmic agent used in the treatment of ventricular arrhythmias and the suppression of atrial and ventricular arrhythmias. The chemical name for amiodarone is 2-butyl-3-benzofuranyl 4-[2-(diethylamino)-ethoxyl]-3,5-diiodophenyl ketone hydrochloride.
| Contents |
History
Amiodarone was initially developed in 1961 in Belgium as a treatment for angina. It was widely used throughout Europe as an anti-anginal medication, and was soon found to suppress arrhythmias.
Dr. Bramah Singh determined that amiodarone and sotalol belonged to a new class of antiarrhythmic agents (what would become the class III antiarrhythmic agents) that would prolong repolarization of the cardiac action potential. Based on this, the Argentinian physician Dr. Mauricio Rosenbaum began using amiodarone to treat his patients who suffered from supraventricular and ventricular arrhythmias, with impressive results. Based on papers written by Dr. Rosenbaum, physicians in the United States began prescribing amiodarone to their patients with potentially life-threatening arrhythmias in the late 1970s. By that time, amiodarone was commonly prescribed throughout Europe for the treatment of arrhythmias. Because amiodarone was not approved by the FDA for use in the United States at the time, physicians were forced to directly obtain amiodarone from pharmaceutical companies in Canada and Europe.
The FDA was reluctant to officially approve the use of amiodarone, since initial reports had shown increased incidence of serious pulmonary side-effects of the drug. In the mid 1980s, the European pharmaceutical companies began putting pressure on the FDA to approve amiodarone by threatening to cut the supply to the American physicians if it was not approved. In December of 1985, amiodarone was approved by the United States FDA for the treatment of arrhythmias. This makes amiodarone one of the few drugs approved by the FDA without rigorous randomized clinical trials.
Dosing
Amiodarone is available in oral and intravenous formulations. Orally, it is available under the trade names Pacerone® (produced by Upsher-Smith Laboratories, Inc.) and Cordarone® (produced by Wyeth-Ayerst Laboratories) in 200 mg and 400 mg tablets.
The dose of amiodarone administered is tailored to the individual and the arrhythmia that is being treated. When administered orally, the bioavailability of amiodarone is quite variable. Absorption ranges from 22 to 95%, with better absorption when it is given with food.1
Amiodarone is fat-soluble, and tends to concentrate in tissues including fat, muscle, liver, lungs, and skin. This confers a high volume of distribution (5000 liters in a 70kg adult) and a long half-life. Due to the long half-life of amiodarone, oral loading typically takes days to weeks.
An oral loading dose is typically a total of 10 grams, divided over one to two weeks. Once an individual is loaded, a typical maintenance dose of amiodarone is 100 or 200 mg either once or twice daily.
Mechanism of action
Amiodarone is categorized as a class III antiarrhythmic agent, and prolongs phase 3 of the cardiac action potential. It has numerous other effects however, including actions that are similar to those of antiarrhythmic classes Ia, II, and IV.
Amiodarone shows beta blocker-like and calcium channel blocker-like actions on the SA and AV nodes, increases the refractory period via sodium- and potassium-channel effects, and slows intra-cardiac conduction of the cardiac action potential, via sodium-channel effects.
Indications for use
Because amiodarone has a low incidence of pro-arrhythmic effects, it has been used both in the treatment of acute life-threatening arrhythmias as well as the chronic suppression of arrhythmias. It is useful both in supraventricular arrhythmias and ventricular arrhythmias.
Ventricular fibrillation
The treatment of choice for ventricular fibrillation (VF) is electrical defibrillation. However amiodarone can be useful in shock-refractory VF. In the ARREST trial, amiodarone was shown to improve survival to hospital admission (when compared to placebo) in individuals who suffer cardiac arrest with shock-refractory VF.2 It is on the basis of this study that the guidelines created by the American Heart Association for the treatment of VF include amiodarone as a second line agent (after epinephrine or vasopressin). ARREST was not adequately powered to demonstrate survival to hospital discharge.
Ventricular tachycardia
Amiodarone may be used in the treatment of ventricular tachycardia in certain instances. Individuals with hemodynamically unstable ventricular tachycardia should not initially receive amiodarone. These individuals should be defibrillated out of their unstable rhythm.
Amiodarone can be used in individuals with hemodynamically stable ventricular tachycardia. In these cases, amiodarone can be used regardless of the individual's underlying heart function and the type of ventricular tachycardia; it can be used in individuals with monomorphic ventricular tachycardia as well as individuals with polymorphic ventricular tachycardia. The dose of amiodarone is 150 mg IV administered over 10 minutes.
Atrial fibrillation
Individuals who have undergone open heart surgery are at an increased risk of developing atrial fibrillation (or AF) in the first few days post-procedure. In the ARCH trial, intravenous amiodarone (2 grams administered over 2 days) has been shown to reduce the incidence of atrial fibrillation after open heart surgery when compared to placebo.3 However, clinical studies have failed to demonstrate long-term efficacy and have shown potentially fatal side effects such as pulmonary toxicities. While Amiodarone is not approved for AF by the FDA, it is a commonly prescribed off-label treatment due to the lack of efficacious treatment alternatives.
Contraindications
The only absolute contraindications to the administration of amiodarone is allergic reaction (ie: anaphylaxis) to the compound. However, because of the wide spectrum of the mechanism of action of amiodarone and the numerous side effects possible, there are a number of groups for which care should be taken when administering the drug.
Individuals who are pregnant or may become pregnant are strongly advised to not take amiodarone. Since amiodarone can be expressed in breast milk, women taking amiodarone are advised to stop nursing.
It is contraindicated in individuals with sinus nodal bradycardia, atrioventricular block, and second or third degree heart block who do not have an artificial pacemaker.
Individuals with baseline depressed lung function should be monitored closely if amiodarone therapy is to be initiated.
Metabolism
Amiodarone is extensively metabolized in the liver, and can effect the metabolism of numerous other drugs. The major metabolite of amiodarone is desethylamiodarone (DEA), which also has antiarrhythmic properties. The metabolism of amiodarone is inhibited by grapefruit juice, leading to elevated serum levels of amiodarone.
Interactions with other drugs
The pharmacokinetics of numerous drugs, including many that are commonly administered to individuals with heart disease, are effected by amiodarone. Particularly, doses of digoxin should be halved in individuals taking amiodarone.
Amiodarone potentiates the action of warfarin. Individuals taking both of these medications should have their warfarin dose halved and their anticoagulation status (measured as prothrombin time (PT) and international normalized ratio (INR)) measured more frequently. The effect of amiodarone in the warfarin concentration can be as early as a few days after initiation of treatment, or can be delayed a few weeks.
Amiodarone inhibits the action of the cytochrome P450 isozyme family. This reduces the clearance of many drugs, including the following: -
Excretion
Unlike most other drugs, which are excreted via the urine or feces, amiodarone is excreted via shedding of epithelial cells. This includes loss of skin cells and loss of the cells of the lining of the gastrointestinal system. While the human body sheds millions of cells a day, the amount of amiodarone lost per day is small, giving a long half life (13 to 103 days). Therefore, if an individual was taking amiodarone on a chronic basis, if it is stopped it will remain in the system for months.
Side effects
Amiodarone has numerous side effects. Most individuals administered amiodarone on a chronic basis will experience at least one side effect.
Thyroid
Due to the iodine content of the agent (37.3% by weight), abnormalities in thyroid function are common. Amiodarone is structurally similar to thyroxine (a thyroid hormone), which contributes to the effects of amiodarone on thyroid function. The incidence of hypothyroidism is about 6%, while the incidence of hyperthyroidism is about 2%. They are called Wolff-Chalkoff and Jodbasedow effect separately.
Eye
Corneal micro-deposits are almost universally present (over 90%) in individuals taking amiodarone for at least 6 months. These deposits typically do not cause any symptoms. About 1 in 10 individuals may complain of a blueish halo.
Gastrointestinal system
Liver toxicity due to amiodarone is quite rare. A drug-induced hepatitis (inflammation of the liver) may occur and is sometimes reversible by lowering the dose.
Skin
Long-term administration of amiodarone is associated with a blue-grey discoloration of the skin. This is more commonly seen in individuals with lighter skin tones. The discoloration may revert upon cessation of the drug. However, the skin color may not return completely to normal.
Individuals taking amiodarone may become more sensitive to the harmful effects of UV-A light. Taking sunblock that also blocks UV-A rays appears to prevent this side effect.
Lung
The most serious reaction that is due to amiodarone is idiopathic pulmonary fibrosis. The incidence of pulmonary fibrosis is not dose related. Some individuals were noted to develop pulmonary fibrosis after a week of treatment, while others did not develop it after years of continuous use. There are no known factors that increase the incidence of amiodarone-induced pulmonary fibrosis in a particular individual. Common practice is to avoid the agent if possible in individuals with decreased lung function.
The most specific test of pulmonary toxicity due to amiodarone is a dramatically decreased DLCO noted on pulmonary function testing.
Related topics
- Advanced cardiac life support (ACLS)
- Antiarrhythmic agents
- Atrial fibrillation
- Cardiac action potential
- Ventricular tachycardia
References
- Siddoway LA. Amiodarone: Guidelines for Use and Monitoring. American Family Physician Dec. 1, 2003. (Full text (http://www.aafp.org/afp/20031201/2189.html))
- Kudenchuk PJ, Cobb LA, Copass MK, Cummins RO, Doherty AM, Fahrenbruch CE, Hallstrom AP, Murray WA, Olsufka M, Walsh T. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999 Sep 16;341(12):871-8. (Medline abstract (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10486418))
- Guarnieri T, Nolan S, Gottlieb SO, Dudek A, Lowry DR. Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: the Amiodarone Reduction in Coronary Heart (ARCH) trial. J Am Coll Cardiol. 1999 Aug;34(2):343-7. (Medline abstract (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10440143))
External links
- Amiodarone (MedicineNet.com) (http://www.medicinenet.com/amiodarone/article.htm)
- Amiodarone (FamilyPracticeNotebook.com) (http://www.fpnotebook.com/CV192.htm)
- Iodine-induced thyrotoxicosis (http://www.thyroidmanager.org/Chapter13/Ch-13-5.htm)
- Amiodarone (Tiscali.com) (http://tiscali.medicdirect.co.uk/tests/default.asp?step=4&pid=1561)
- Amiodarone (The WorldWide Intensivist) (http://www.anaesthetist.com/icu/manage/drugs/heart/amiodarone.htm)
- pt:Amiodarona